abbott point of care covid test
The Afinion 2 System is a global market leader in highly accurate point-of-care POC testing for physician offices and clinics. Our first molecular test is.
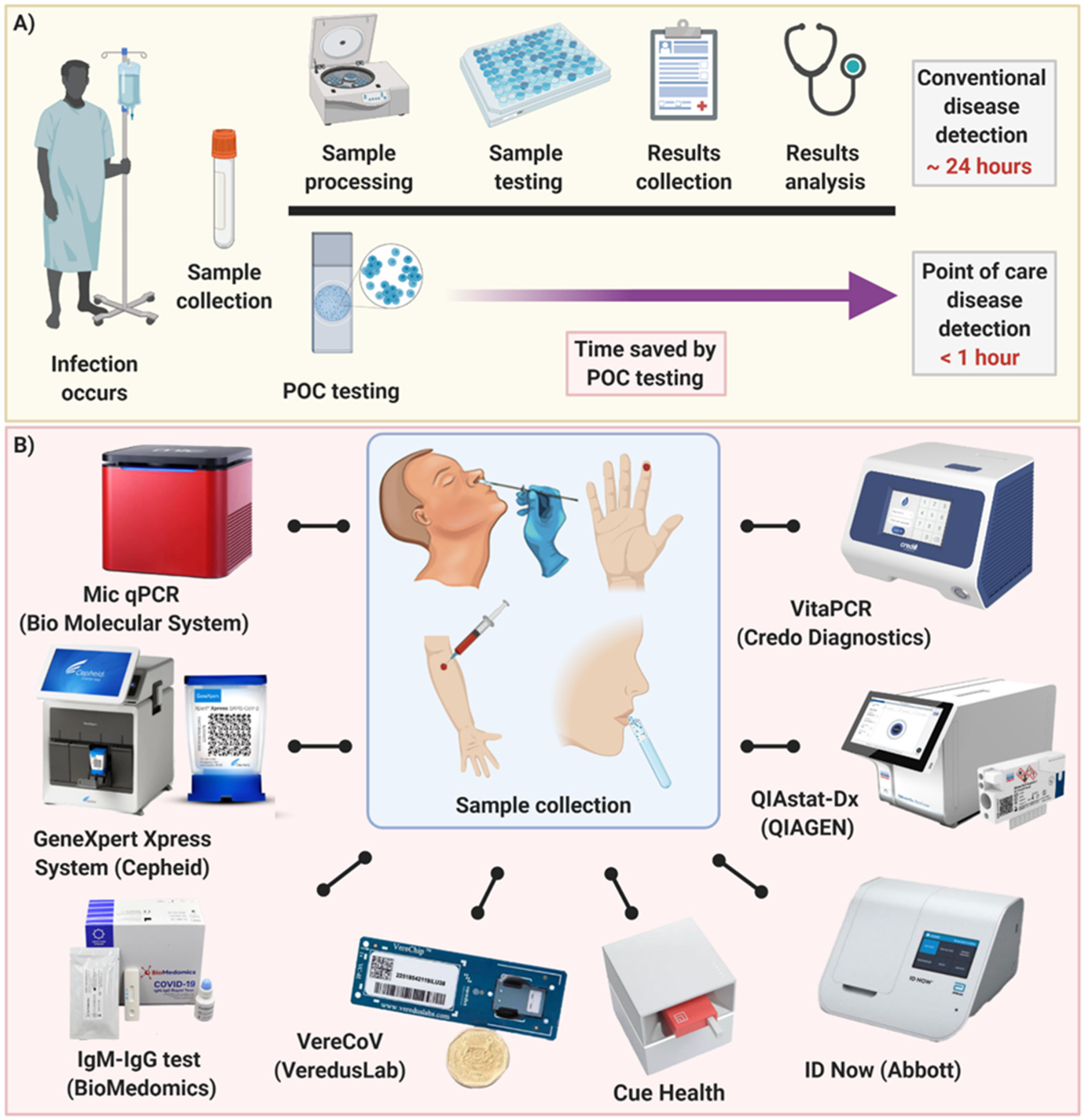
Diagnostics Free Full Text Point Of Care Diagnostics In The Age Of Covid 19 Html
A CLIA Certificate of Waiver is appropriate if SARS-CoV-2 point-of-care testing is the only testing being.
. Food and Drug Administration Emergency Use Authorization EUA. A CLIA certificate is required to perform point-of-care testing. The Abbott Panbio COVID-19 Antigen Self Test rapidly and reliably detects acute corona infection within 15 minutes.
With the Afinion 2 System we take the complexity out of the. We have molecular and antigen tests that help detect an active infection of COVID-19 as well as serology tests that help detect antibodies. Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes.
The portable rapid molecular ID NOW COVID-19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations. It is used as a molecular point-of-care platform for Influenza A and B Strep A and RSV tests. In the case of COVID-19 point-of-care tests have become critical because of their portability speed and reliability.
The COVID-19 pandemic will be fought on multiple fronts and a portable. Point-of-care antigen tests are an important tool for SARS-CoV-2 detection yet are less clinically sensitive than real-time reverse-transcription PCR RT-PCR impacting their efficacy as. The ID NOW COVID-19 assay is now available for use on the ID NOW platform under US.
The ID NOW COVID-19. The test has been authorized only for the detection of. It has been authorized by the FDA under an emergency use authorization for use by authorized laboratories and patient care settings.
Abbott ABT launches a portable molecular point-of-care test to speed up coronavirus detection especially in areas with severe outbreaks. The BinaxNOW COVID-19 Ag Card 2 Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal. The Food and Drug Administration FDA has issued an Emergency Use.
Our ID NOW COVID-19 rapid point-of-care test can provide. The Abbott ID NOW COVID-19 tests runs on the ID NOW platform and delivers results within minutes. The individually packaged and filled extraction solutions make it.
Abbotts molecular point-of-care test for COVID-19 delivers positive results in as little as five minues and negative results in 13 minutes.

Covid 19 Point Of Care Diagnostics Present And Future Acs Nano

Covid 19 Point Of Care Diagnostics Present And Future Acs Nano

Abbott S 5 Covid 19 Rapid Antigen Test Gets Emergency Use Status From Fda Wsj

Abbott Stock Soars On News Of 5 Minute Test For Covid 19 Barron S

Abbott Launches Molecular Point Of Care Test To Detect Novel Coronavirus In As Little As Five Minutes Mar 27 2020

Abbott Realtime Sars Cov 2 Assay Eua Abbott Molecular

Covid News Walgreens Cvs Limits How Many At Home Tests Customers Can Buy
Primer Arizona Continues To Ramp Up Testing Office Of The Arizona Governor

Binaxnow Covid 19 Antigen Self Test Abbott Point Of Care

Abbott Id Now Covid 19 Instructions Modified
Rapid Covid 19 Testing Breaks Free From The Lab
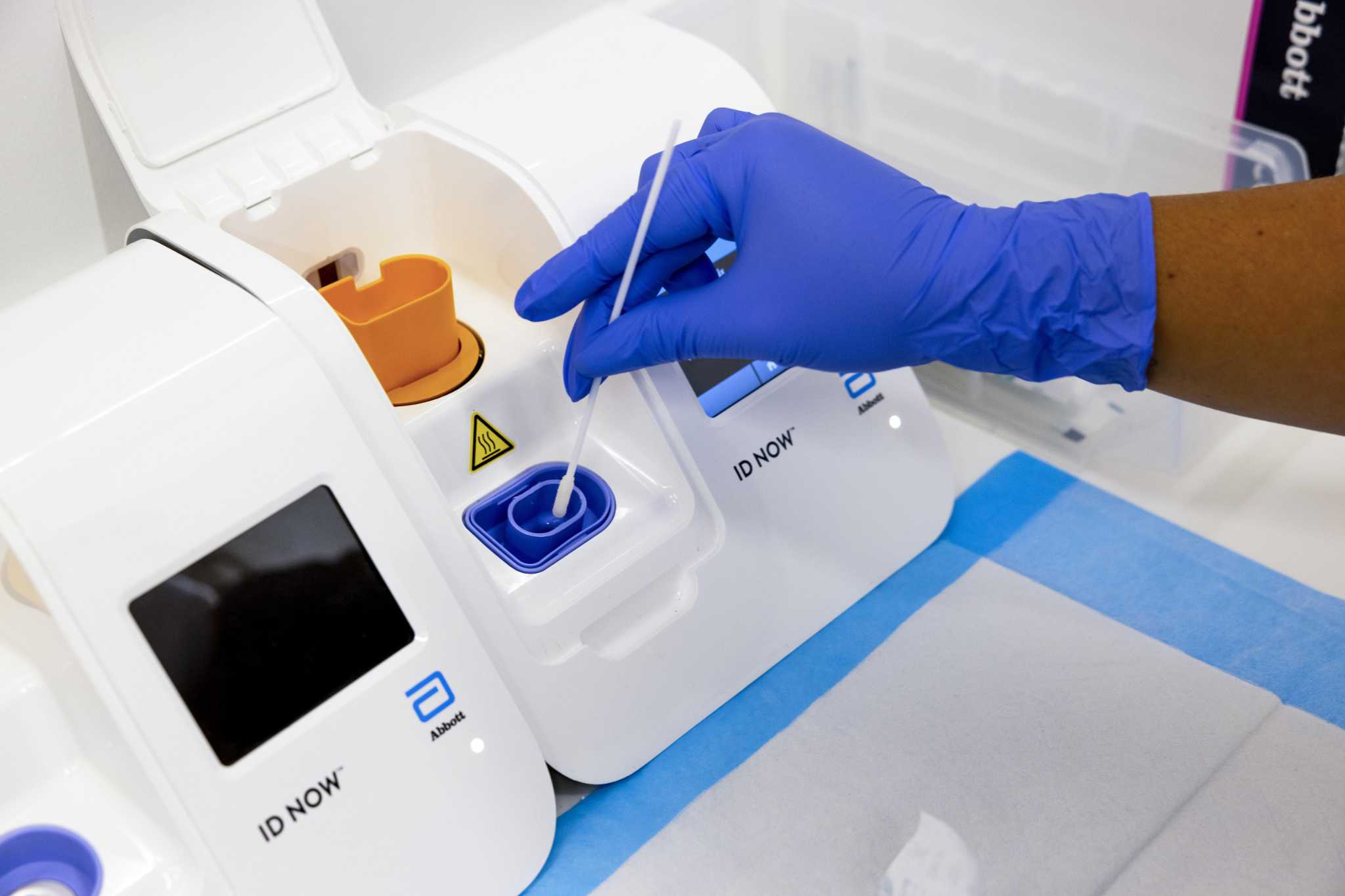
Some Bay Area Hospitals Return Covid Tests Results Within An Hour Here S Why It S Not More Common
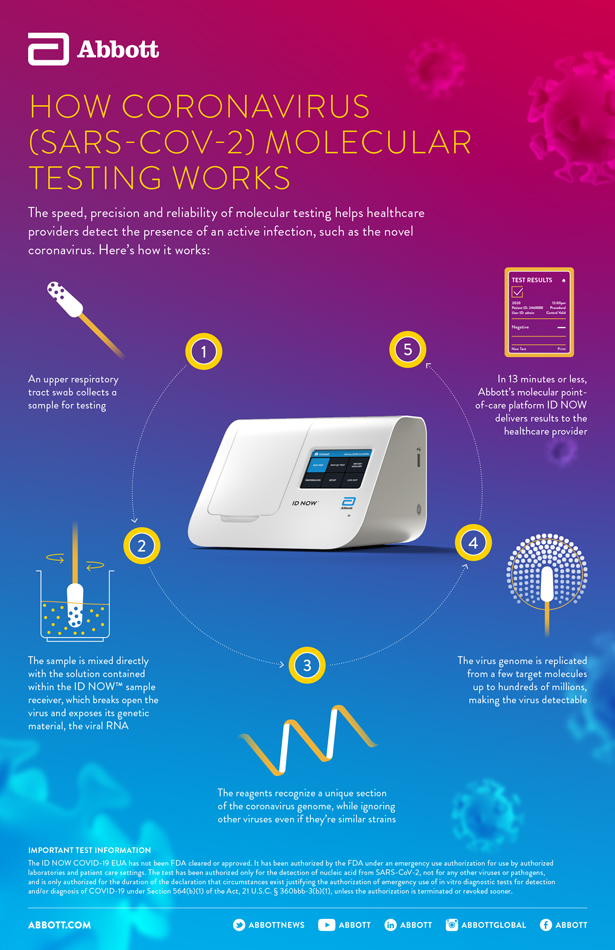
How Id Now Tackles Covid 19 Abbott Newsroom

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News
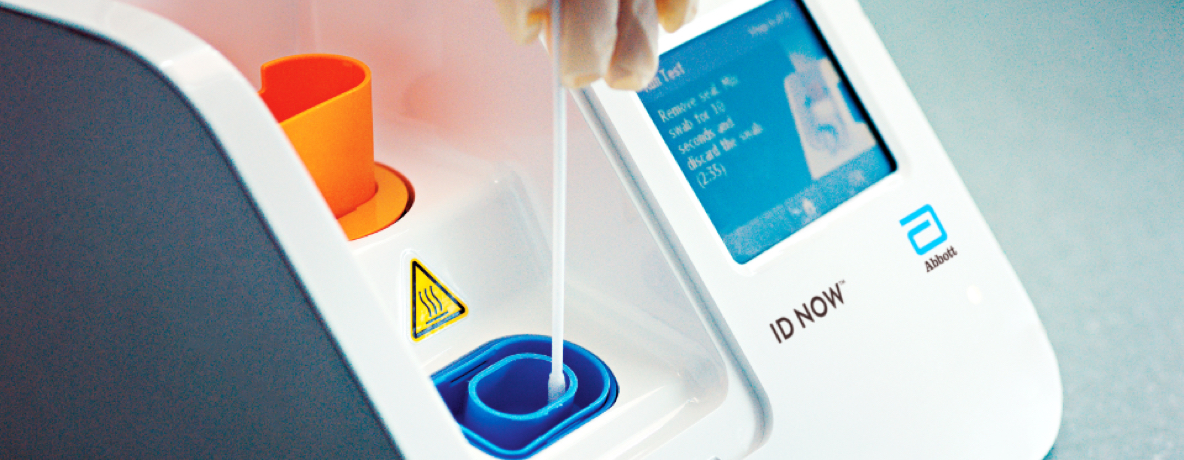
Covid 19 Testing The Center For Urgent Care
Abbott S Point Of Care Covid 19 Test Detects Coronavirus In As Little As 5 Minutes Biospace